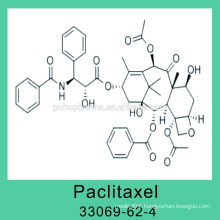
Vinorelbine Tartrate with GMP 125317-39-7 NVB Best Quality in China
Basic Info
Model No.: USP35
Product Description
Test Item | Specifications | Result |
Apperance | White to yellow or light brown, amorphous powder | white powder |
Solubility | Freely soluble in water | Complies |
Color of solution | The solution is clear | Complies |
| Identification A: Infrared absorption B: RT of main peak C: Reaction of tartrates | Complies to the reference standard Complies to the reference standard Positive | Complies Complies Complies |
pH | 3.3 ~ 3.8 | 3.4 |
Residue on ignition | ≤0.1% | 0.01% |
Water | ≤4.0% | 2.2% |
| Related Substances 3,6-Epoxy vinorelbine Vinorelbine Impurity A Any individual impurity Total impurities |
≤0.3% (3,6-Epoxy vinorelbine) ≤0.2% (Vinorelbine Impurity A) ≤0.2% (Any individual impurity) ≤0.7% (Total impurities) | Not found Not found 0.06% 0.2% |
| Residual Solvent
Acetone Methanol Methylene chloride Chloroform Tetrahydrofuran Ethanol | ≤0.5% ≤0.3% ≤0.006% ≤0.072% ≤0.5% ≤0.5% |
0.354% 0.045% 0.0004% Not found Not found 0.037% |
| Microbial Limit Bacteria Fungi Escherichia coli |
≤800cfu/g ≤80cfu/g Not Found/g |
20cfu/g 20cfu/g Not Found/g |
Assay | 98.0%~102.0% | 99.8% |
Conclusion: Complies with all requirements of the USP35 standards of Vinorelbine Tartrate | ||
 We look forward to meeting you!!!
We look forward to meeting you!!!  Delivery Can we provide sample? Yes, we can provide FREE sample for most products. We are sincerely to cooperate with your esteemed company.
Delivery Can we provide sample? Yes, we can provide FREE sample for most products. We are sincerely to cooperate with your esteemed company.  Please don't hesitate to contact me!!!
Please don't hesitate to contact me!!!  Contact us if you need more details on Vinorelbine Tartrate. We are ready to answer your questions on packaging, logistics, certification or any Other aspects about 125317-39-7、Vinorelbine. If these products fail to match your need, please contact us and we would like to provide relevant information.
Contact us if you need more details on Vinorelbine Tartrate. We are ready to answer your questions on packaging, logistics, certification or any Other aspects about 125317-39-7、Vinorelbine. If these products fail to match your need, please contact us and we would like to provide relevant information. Product Categories : API > Antineoplastic